 Guideline Essentials
Guideline Essentials
Uveal melanoma affects the choroid, iris or ciliary body and is a rare but aggressive form of malignant melanoma with a poor prognosis in the metastatic disease.
Treatment requires a multidisciplinary approach, including primary therapy, molecular diagnostics, follow-up care, and if necessary, medical treatment and liver-directed procedures.
*Surgery: Large tumors and those with scleral or extrascleral spread are usually treated by enucleation or, if necessary, irradiated with protons.
*Radiotherapy: Smaller tumors can be treated with brachytherapy or proton irradiation. Radiotherapeutic procedures show similar tumor healing rates and have the advantage of preserving the bulb compared to enucleation.
*Iris melanoma: Important risks associated with iris melanomas include secondary glaucoma and cataract formation as well as increased susceptibility to glare; smaller iris melanomas can also be resected if necessary.
However, an uveal melanoma is often a distinct clinical diagnosis, i.e. taking a biopsy for histology and possibly molecular genetics must be considered, as
there is a risk of complications such as retinal detachment, bleeding or infection.
molecular genetic examinations are not desired by all patients, e.g. determination of chromosome 3 status (monosomy 3 vs. disomy 3). This information is crucial for the prognosis and planning of follow-up care.
molecular genetic examinations can be obtained from the blood using liquid biopsy.
Follow-up includes:
Ophthalmologic examination: e.g. local recurrence
Liver ultrasound: e.g. liver metastases
Lab: transaminases, cholestasis parameters, LDH and S100
In case of unclear findings: MRI of the liver and further sectional imaging
Recommendations should ideally be based on the risk profile of the tumor:
High-risk profile (large tumors, from stage IIIB, monosomy 3): Examinations every 3 months for the first 3-5 years
Low risk: examinations every 6 months
As many patients do not receive a genetic examination (e.g. for monosomy 3), a follow-up examination should be carried out every 3 months in case of doubt.
*Clinical trials: For patients with uveal melanoma, participation in clinical trials on new therapeutic approaches is an important option.
*Tebentafusp: Tebentafusp is a bispecific fusion protein and the first approved systemic therapy for HLA-A02:01-positive patients with inoperable or metastatic uveal melanoma.
Tebentafusp has significantly improved overall survival in clinical trials compared to standard therapy.
The drug is administered intravenously and requires careful monitoring for side effects, particularly cytokine release syndrome (CRS) and skin reactions.
*Immune checkpoint inhibitors (ICIs): ICIs such as ipilimumab and nivolumab can be used in combination or as a single therapy. However, the efficacy of ICIs in uveal melanoma is lower compared to cutaneous melanoma.
Other therapies: For patients who do not respond to tebentafusp or ICIs, other treatment options such as chemotherapy or targeted therapies are available.
The study situation on liver-directed procedures in uveal melanoma is limited and the results are inconsistent.
In some studies, local liver procedures showed an improvement in PFS and response rates compared to systemic therapy, but no benefit in overall survival.
Liver-directed procedures may be considered in patients with metastatic uveal melanoma, especially if there is a high tumor burden in the liver.
These procedures include:
*Hepatic chemosaturation/chemoperfusion involves isolated perfusion of the liver to deliver a high concentration of chemotherapeutic agents directly into the tumor.
*Selective Internal Radiotherapy (SIRT): Radioactive microspheres are injected into the tumor via the hepatic artery.
*Transarterial chemoembolization (TACE): A combination of chemotherapeutic agents and embolization material is injected into the hepatic artery to cut off the tumour from the blood supply.
*Resection: Surgical removal of liver metastases is only possible for isolated metastases. As the liver is often diffusely infiltrated in uveal melanomas, resection is rarely an option.The recurrence rate after resection is high.
The choice of the optimal local liver procedure (e.g. chemoperfusion vs. TACE vs. SIRT) depends on various factors, such as the size and location of the metastases, liver function and the patient's general condition.
Damato BE, Dukes J, Goodall H, Carvajal RD. Tebentafusp: T cell redirection for the treatment of metastatic uveal melanoma. Cancers 2019;11. https://doi.org/10.3390/cancers11070971.2
Dieser Artikel bietet einen Überblick über Tebentafusp und seine Anwendung bei der Behandlung des metastasierten Uveamelanoms. Er beschreibt den Wirkmechanismus von Tebentafusp, die Ergebnisse klinischer Studien und das Sicherheitsprofil des Medikaments.3
Boudousquie C, Bossi G, Hurst JM, Rygiel KA, Jakobsen BK, Hassan NJ. Polyfunctional response by ImmTAC (IMCgp100) redirected CD8(+) and CD4(+) T cells. Immunology 2017;152:425–38. https://doi.org/10.1111/imm.12779].24
Diese Studie untersucht die polyfunktionelle Reaktion von durch ImmTAC (IMCgp100) umgeleiteten CD8+ und CD4+ T-Zellen. Sie zeigt, dass Tebentafusp die Aktivierung und Proliferation von T-Zellen fördert, die dann Tumorzellen abtöten können.5
Kimmtrak Summary of Product Characteristics. 2022.6789...
Die Fachinformation von Kimmtrak enthält detaillierte Informationen über das Medikament, einschliesslich der Indikationen, Dosierung, Art der Anwendung, Gegenanzeigen, Warnhinweise, Nebenwirkungen, pharmakologischen Eigenschaften und weitere relevante Aspekte.4
Berman DM, Bell JI. Redirecting polyclonal T cells against cancer with soluble T cell receptors. Clin Cancer Res 2022;29:697–704. https://doi.org/10.1158/1078-0432.Ccr-22-0028].2
In diesem Artikel wird die Verwendung von löslichen T-Zell-Rezeptoren (TCRs) zur Umleitung polyklonaler T-Zellen gegen Krebs erörtert. Tebentafusp ist ein Beispiel für ein solches lösliches TCR-basiertes Medikament, das die Immunantwort gegen Tumorzellen verstärkt.14
Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 2021;385:1196–206. https://doi.org/10.1056/NEJMoa2103485].261415
Diese bahnbrechende Studie zeigte, dass Tebentafusp das Gesamtüberleben von Patienten mit metastasiertem Uveamelanom im Vergleich zur Standardtherapie signifikant verbessert. Sie bildete die Grundlage für die Zulassung von Tebentafusp für diese Indikation.6
Carvajal RD, Butler MO, Shoushtari AN, Hassel JC, Ikeguchi A, Hernandez-Aya L, et al. Clinical and molecular response to tebentafusp in previously treated patients with metastatic uveal melanoma: a phase 2 trial. Nat Med 2022;28:2364–73. https://doi. org/10.1038/s41591-022-02015-7].261516...
In dieser Phase-II-Studie wurde Tebentafusp bei Patienten mit vorbehandeltem metastasiertem Uveamelanom untersucht. Die Ergebnisse zeigten vielversprechende klinische und molekulare Ansprechraten, was darauf hindeutet, dass Tebentafusp auch in späteren Therapielinien wirksam sein kann.24
Sullivan RJ, Milhem MM, Demidov LV, Lewis KD, Schlaak M, Piperno-Neumann S, et al. Treatment with tebentafusp beyond radiographic progressive disease (PD) in metastatic uveal melanoma (mUM). J Clin Oncol 2022;40:9585. https://doi.org/10. 1200/JCO.2022.40.16_suppl.9585].25
Diese Studie untersuchte die Fortsetzung der Tebentafusp-Therapie über das radiologische Fortschreiten der Erkrankung hinaus bei Patienten mit metastasiertem Uveamelanom. Sie lieferte Hinweise darauf, dass Patienten auch bei radiologischem Progress von der Therapie profitieren können, insbesondere wenn sie eine Reduktion der zirkulierenden Tumor-DNA (ctDNA) zeigen.26
Rantala ES, Hernberg MM, Piperno-Neumann S, Grossniklaus HE, Kivelä TT. Metastatic uveal melanoma: the final frontier. Prog Retin Eye Res 2022;90:101041. https://doi.org/10.1016/j.preteyeres.2022.101041].724272829
Dieser Übersichtsartikel befasst sich mit den Herausforderungen und Fortschritten bei der Behandlung des metastasierten Uveamelanoms. Er beleuchtet die genetischen Unterschiede zum kutanen Melanom, die Bedeutung lebergerichteter Therapien und den Bedarf an wirksamen systemischen Therapien wie Tebentafusp.30
Mahendraraj K, Shrestha S, Lau CS, Chamberlain RS. Ocular melanoma-when you have seen one, you have not seen them all: a clinical outcome study from the Surveillance, Epidemiology and End Results (SEER) database (1973-2012). Clin Ophthalmol 2017;11:153–60. https://doi.org/10.2147/opth.S120530].25
Diese Studie analysierte die klinischen Ergebnisse von Patienten mit okulärem Melanom anhand der SEER-Datenbank. Sie liefert Erkenntnisse zur Epidemiologie, zum natürlichen Verlauf und zur Prognose des okulären Melanoms, was wichtig für die Entwicklung und Bewertung neuer Therapien ist.15
Kaliki S, Shields CL. Uveal melanoma: relatively rare but deadly cancer. Eye 2017;31:241–57. https://doi.org/10.1038/eye.2016. 275].242831
Dieser Artikel bietet einen umfassenden Überblick über das Uveamelanom, einschliesslich Epidemiologie, Risikofaktoren, klinischer Präsentation, Diagnostik, Prognose und Behandlungsoptionen. Er unterstreicht die Bedeutung der Früherkennung und die Herausforderungen bei der Behandlung der metastasierten Erkrankung.32
Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol 2017;101:38–44. https://doi.org/10.1136/bjophthalmol-2016-309034].243133
Dieser Artikel befasst sich mit den Behandlungsoptionen für Patienten mit metastasiertem Uveamelanom. Er beschreibt etablierte Therapien wie lebergerichtete Verfahren und systemische Therapien und diskutiert neue Ansätze und zukünftige Perspektiven, einschliesslich der Entwicklung von zielgerichteten Therapien und Immuntherapien.34
Nathan P, Cohen V, Coupland S, Curtis K, Damato B, Evans J, et al. Uveal melanoma UK national guidelines. Eur J Cancer 2015;51:2404–12. https://doi.org/10.1016/j.ejca.2015.07.013].2431
Diese nationalen Leitlinien aus Grossbritannien bieten Empfehlungen zur Diagnose, Behandlung und Nachsorge von Patienten mit Uveamelanom. Sie dienen als Grundlage für die klinische Praxis und tragen zur Standardisierung der Versorgung bei.16
Heppt MV, Steeb T, Schlager JG, Rosumeck S, Dressler C, Ruzicka T, et al. Immune checkpoint blockade for unresectable or metastatic uveal melanoma: a systematic review. Cancer Treat Rev 2017;60:44–52. https://doi.org/10.1016/j.ctrv.2017.08.009].2435
Dieser systematische Review fasst die Evidenz zur Wirksamkeit von Immuncheckpoint-Inhibitoren bei der Behandlung des nichtresektablen oder metastasierten Uveamelanoms zusammen. Er zeigt das Potenzial dieser Therapien auf, unterstreicht aber auch die Notwendigkeit weiterer Forschung, um ihre optimale Anwendung in dieser Indikation zu ermitteln.36
Kirchberger MC, Hauschild A, Schuler G, Heinzerling L. Combined low-dose ipilimumab and pembrolizumab after se-quential ipilimumab and pembrolizumab failure in advanced melanoma. Eur J Cancer 2016;65:182–4. https://doi.org/10.1016/j. ejca.2016.07.003].35
Diese Studie untersuchte die kombinierte Anwendung von niedrigdosiertem Ipilimumab und Pembrolizumab bei Patienten mit fortgeschrittenem Melanom, die auf eine sequenzielle Therapie mit diesen Medikamenten nicht angesprochen hatten. Die Ergebnisse deuten darauf hin, dass diese Kombinationstherapie eine wirksame Option für diese Patienten sein kann.7
Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer 2018;6:56. https://doi.org/10.1186/s40425- 018-0343-9].2130
Dieser Artikel gibt einen Überblick über das Zytokin-Freisetzungssyndrom (CRS), eine potenziell lebensbedrohliche Nebenwirkung, die bei einigen Immuntherapien auftreten kann. Er beschreibt die Pathophysiologie, die klinischen Symptome, die Diagnostik und das Management des CRS.37
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune ef-fector cells. Biol Blood Marrow Transplant 2019;25:625–38. https://doi.org/10.1016/j.bbmt.2018.12.758].213238
Dieser Artikel beschreibt die Konsensus-Richtlinien der American Society for Transplantation and Cellular Therapy (ASTCT) zur Graduierung des CRS und der Neurotoxizität, die mit Immuntherapie assoziiert sind. Diese Richtlinien dienen der Standardisierung der Bewertung und des Managements dieser Nebenwirkungen.22
Piulats Rodriguez JM, Piperno-Neumann S, Rutkowski P, Nathan P, Hasse JC, Espinosa E, et al. A propensity score weighted comparison of tebentafusp or pembrolizumab versus combination ipilimumab and nivolumab in untreated metastatic uveal melanoma. Ann Oncol 2022;33:S356–409. https://doi.org/ 10.1016/j.annonc.2022.07.949].21
Diese Studie verglich die Wirksamkeit von Tebentafusp oder Pembrolizumab mit der Kombination von Ipilimumab und Nivolumab bei Patienten mit unbehandeltem metastasiertem Uveamelanom. Sie lieferte Daten zur vergleichenden Wirksamkeit dieser Therapien in der Erstlinientherapie.39
RoActemra Summary of Product Characteristics. 2021.2240
Die Fachinformation von RoActemra (Tocilizumab) enthält detaillierte Informationen über dieses Medikament, das zur Behandlung des CRS eingesetzt wird.41
Schlaak M, Dummer R, Kirkwood JM, Joshua A, Milhem M, Gastaud L, et al. Safety and efficacy of infrequent tebentafusp treatment omissions in patients with metastatic uveal melanoma. Ann Oncol 2022;33:S356–409. https://doi.org/10.1016/annonc/ annonc59.4042
Diese Studie untersuchte die Sicherheit und Wirksamkeit von gelegentlichen Auslassungen der Tebentafusp-Behandlung bei Patienten mit metastasiertem Uveamelanom. Sie zeigte, dass gelegentliche Auslassungen in der Regel gut vertragen werden und keinen negativen Einfluss auf die Wirksamkeit haben.42
Bechrakis NE, Bornfeld N, Heindl LM, Skoetz N, Leyvraz S, Joussen AM. Das uveale Melanom – standardisiertes Vorgehen in Diagnostik, Therapie und Nachsorge. Klin Monbl Augenheilkd 2021;238:761–72. https://doi.org/10.1055/a-1534- 0198].182940
Dieser Artikel beschreibt ein standardisiertes Vorgehen für die Diagnose, Therapie und Nachsorge von Patienten mit Uveamelanom in Deutschland. Er dient als Leitfaden für die klinische Praxis und trägt zur Verbesserung der Patientenversorgung bei.17
Pereira FH, Batalhão ME, Cárnio EC. Correlation between body temperature, blood pressure and plasmatic nitric oxide in septic patients. Rev Lat Am Enfermagem 2014;22:123–8. https://doi. org/10.1590/0104-1169.2896.2392].4344
Diese Studie untersuchte den Zusammenhang zwischen Körpertemperatur, Blutdruck und Plasma-Stickstoffmonoxid bei septischen Patienten. Sie liefert Erkenntnisse zur Pathophysiologie des septischen Schocks, die relevant für das Verständnis des CRS sein können.45
Salama AKS, Cheshuk V, Siveke J, Berrocal A, Abdullah SE, Lockwood S, et al. Characterization of cytokine release syndrome (CRS) following treatment with tebentafusp in previously un-treated patients with metastatic uveal melanoma. Ann Oncol 2021;32:S829–66. https://doi.org/10.1016/j.annonc.2021.08.1398].1144
Diese Studie charakterisierte das CRS bei Patienten mit vorbehandeltem metastasiertem Uveamelanom, die mit Tebentafusp behandelt wurden. Sie lieferte detaillierte Informationen über die Häufigkeit, den Schweregrad und das Management des CRS in dieser Patientengruppe.18
Stein A, Franklin JL, Chia VM, Arrindell D, Kormany W, Wright J, et al. Benefit-risk assessment of blinatumomab in the treatment of relapsed/refractory B-cell precursor acute lympho-blastic leukemia. Drug Saf 2019;42:587–601. https://doi.org/10. 1007/s40264-018-0760-1].1146
Dieser Artikel bewertet den Nutzen und das Risiko von Blinatumomab bei der Behandlung der rezidivierten/refraktären B-Zell-Vorläufer-akuten lymphatischen Leukämie. Blinatumomab ist ein weiteres Beispiel für ein bispezifisches Antikörper-basiertes Medikament, das CRS auslösen kann.23
Choudhry J, Parson M, Wright J. A retrospective review of to-cilizumab for the management of blinatumomab (a bispecific T cell engager)-induced cytokine release syndrome (CRS). Blood 2018;132:5211. https://doi.org/10.1182/blood-2018-99-117353].1146
Diese retrospektive Studie untersuchte die Verwendung von Tocilizumab zur Behandlung des durch Blinatumomab induzierten CRS. Sie lieferte Evidenz für die Wirksamkeit von Tocilizumab bei der Behandlung dieser Nebenwirkung.19
Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, et al. Cytokine release syndrome after blinatu-momab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013;121:5154–7. https://doi.org/10.1182/blood-2013-02-485623].47
Diese Studie untersuchte die Pathophysiologie des durch Blinatumomab induzierten CRS und zeigte, dass es mit einer abnormalen Makrophagenaktivierung zusammenhängt. Sie zeigte auch, dass eine auf Zytokine gerichtete Therapie, wie z.B. Tocilizumab, das CRS wirksam lindern kann.48
Queudeville M, Handgretinger R, Ebinger M. Immunotargeting relapsed or refractory precursor B-cell acute lymphoblastic leu-kemia - role of blinatumomab. Onco Targets Ther 2017;10: 3567–78. https://doi.org/10.2147/ott.S103470].47
Dieser Artikel befasst sich mit der Rolle von Blinatumomab bei der Behandlung der rezidivierten oder refraktären B-Zell-Vorläufer-akuten lymphatischen Leukämie. Er beschreibt den Wirkmechanismus von Blinatumomab, die Ergebnisse klinischer Studien und das Management von Nebenwirkungen, einschliesslich des CRS.8
Khoja L, Atenafu EG, Suciu S, Leyvraz S, Sato T, Marshall E, et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: an interna-tional rare cancers initiative (IRCI) ocular melanoma study. Ann Oncol 2019;30:1370–80. https://doi.org/10.1093/annonc/mdz176].1349
Diese Meta-Analyse untersuchte das progressionsfreie Überleben und das Gesamtüberleben von Patienten mit metastasiertem Uveamelanom, um Benchmarks für klinische Studien zu erstellen. Sie lieferte wichtige Daten zur Prognose und zum Therapieansprechen in dieser Indikation.50
Carvajal RD, Sacco JJ, Jager MJ, Eschelman DJ, Olofsson Bagge R, Harbour JW, et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol 2023;20:99–115. https://doi. org/10.1038/s41571-022-00714-1].1349
Dieser Übersichtsartikel beschreibt die Fortschritte im klinischen Management des Uveamelanoms. Er befasst sich mit neuen diagnostischen Verfahren, Therapien und Strategien zur Verbesserung der Patientenversorgung, einschliesslich der Rolle von Tebentafusp und anderen zielgerichteten Therapien.51
Dimitriou F, Hassel JC, Orloff M, Hughes I, Kapiteijn E, Mehmi I, et al. Treatment sequence with tebentafusp (tebe) and anti- PD1/ipilimumab (PD1+IPI) in HLA-A2*02:01 patients (pts) with metastatic uveal melanoma (mUM). Ann Oncol 2022;33:S356–409. https://doi.org/10.1016/j.annonc.2022.07.958].1352
Diese Studie untersuchte die optimale Therapiesequenz von Tebentafusp und Anti-PD-1/Ipilimumab bei Patienten mit metastasiertem Uveamelanom. Sie lieferte Daten zur Wirksamkeit und Sicherheit verschiedener Therapiesequenzen.9
Mouriaux F, Servois V, Parienti JJ, Lesimple T, Thyss A, Dutriaux C, et al. Sorafenib in metastatic uveal melanoma: effi-cacy, toxicity and health-related quality of life in a multicentre phase II study. Br J Cancer 2016;115:20–4. https://doi.org/10. 1038/bjc.2016.119].3352
Diese Phase-II-Studie untersuchte die Wirksamkeit, Toxizität und gesundheitsbezogene Lebensqualität von Sorafenib bei Patienten mit metastasiertem Uveamelanom. Sorafenib ist ein Tyrosinkinase-Inhibitor, der in der Vergangenheit als Therapieoption für diese Indikation untersucht wurde.43
Atkinson TM, Hay JL, Shoushtari A, Li Y, Paucar DJ, Smith SC, et al. Relationship between physician-adjudicated adverse events and patient-reported health-related quality of life in a phase II clinical trial (NCT01143402) of patients with metastatic uveal melanoma. J Cancer Res Clin Oncol 2017;143:439–45. https://doi.org/10.1007/s00432-016-2318-x].3353
Diese Studie untersuchte den Zusammenhang zwischen ärztlich beurteilten unerwünschten Ereignissen und patientenberichteter gesundheitsbezogener Lebensqualität in einer Phase-II-Studie mit Patienten mit metastasiertem Uveamelanom. Sie lieferte Daten zum Einfluss der Therapie auf die Lebensqualität der Patienten.27
van der Kooij MK, Speetjens FM, van der Burg SH, Kapiteijn E. Uveal versus cutaneous melanoma; same origin, very distinct tumor types. Cancers ((Basel)) 2019;11:845. https://doi.org/10. 3390/cancers11060845].3353
Dieser Artikel vergleicht das Uveamelanom und das kutane Melanom, zwei unterschiedliche Melanomtypen mit unterschiedlichen genetischen Veränderungen, Metastasierungsmustern und Ansprechen auf die Therapie.10
Bertolotto C. Cutaneous and uveal melanoma: two different cancers in therapeutic needs. C R Biol 2021;344:219–31. https:// doi.org/10.5802/crbiol.63].3354
Dieser Artikel unterstreicht die Unterschiede zwischen kutanem und Uveamelanom im Hinblick auf den therapeutischen Bedarf. Er beschreibt die molekularen Unterschiede, die zu unterschiedlichen Ansprechraten auf Therapien führen, und plädiert für die Entwicklung von therapiespezifischen Behandlungsstrategien.11
Hoefsmit EP, Rozeman EA, Van TM, Dimitriadis P, Krijgsman O, Conway JW, et al. Comprehensive analysis of cutaneous and uveal melanoma liver metastases. J Immunother Cancer 2020:8. https://doi.org/10.1136/jitc-2020-001501].3354
Diese Studie führte eine umfassende Analyse von Lebermetastasen des kutanen und Uveamelanoms durch. Sie lieferte Daten zu den molekularen Unterschieden und den Immunprofilen dieser Metastasen, was wichtig für die Entwicklung zielgerichteter Therapien ist.55
Chattopadhyay C, Kim DW, Gombos DS, Oba J, Qin Y, Williams MD, et al. Uveal melanoma: from diagnosis to treat-ment and the science in between. Cancer 2016;122:2299–312. https://doi.org/10.1002/cncr.29727].3354
Dieser Artikel bietet einen umfassenden Überblick über das Uveamelanom, von der Diagnose über die Behandlung bis hin zu den wissenschaftlichen Grundlagen. Er befasst sich mit den Herausforderungen bei der Behandlung dieser Erkrankung und beleuchtet die neuesten Forschungsergebnisse und zukünftige Perspektiven.12
van Poppelen NM, de Bruyn DP, Bicer T, Verdijk R, Naus N, Mensink H, et al. Genetics of ocular melanoma: insights into genetics, inheritance and testing. Int J Mol Sci 2020;22:336. https://doi.org/10.3390/ijms22010336].3356
Dieser Artikel befasst sich mit der Genetik des okulären Melanoms, einschliesslich der genetischen Veränderungen, der Vererbung und der genetischen Testung. Er liefert wichtige Informationen für die Risikobewertung, die Diagnosestellung und die Entwicklung personalisierter Therapien.28
Ellis JM, Henson V, Slack R, Ng J, Hartzman RJ, Katovich Hurley C. Frequencies of HLA-A2 alleles in five U.S. population groups: predominance of A∗02011 and identification of HLA- A∗0231. Hum Immunol 2000;61:334–40. https://doi.org/10.1016/ S0198-8859(99)00155-X].3356
Diese Studie untersuchte die Häufigkeit von HLA-A2-Allelen in fünf verschiedenen Bevölkerungsgruppen in den USA. Die Ergebnisse zeigten, dass HLA-A*02011 das häufigste Allel ist, was für die Entwicklung von HLA-A2-gerichteten Therapien wie Tebentafusp relevant ist.57
Olivier T, Prasad V. Tebentafusp in first-line melanoma trials: an outperforming outlier. Transl Oncol 2022;20:101408. https://doi. org/10.1016/j.tranon.2022.101408].3356
Dieser Artikel analysiert die Ergebnisse von Studien mit Tebentafusp in der Erstlinientherapie des Melanoms und stellt fest, dass es sich als ein "Outlier" mit überdurchschnittlicher Leistung erwiesen hat. Er diskutiert die Gründe für diese positive Entwicklung und die Bedeutung von Tebentafusp für die Behandlung des Melanoms.
Parameter of high-risk cSCC - for local recurrence or metastasis
Diameter > 2 cm
Localization on lip/ear/temple
Thickness > 6 mm
Invasion beyond subcutaneous fat / bone erosion
Histological type: desmoplastic / poor differentiation
Immunosuppression
PNI (microscopic, symptomatic or radiological)
Positive histological margins
Surgery
a. Complete excision (R0) with histological confirmation of peripheral and deep margins is first line treatment
b. Large tumours or tumours on the head and neck can undergo a punch or incisional biopsy for histological confirmation
c. In cases of positive margins, a re-excision shall be done.
d. SLNB is not recommended
Safety margins
Low-risk cSCC: 5 mm.
cSCC with high-risk factors: clinical safety margin of 6–10 mm or by micrographically controlled surgery (MCS).
MCS should be considered for cSCC in functional/cosmetical sensitive areas
Wound closure
No local flaps until histologically confirmation of R0-status.
Destructive modalities for cSCC
Electrodissection & Curettage, cryotherapy, PDT, and lasers should not be performed in the treatment of primary invasive cSCC. Exceptions can be considered in small-sized and/or multiple cSCCs in low-risk areas where surgery and/or RT are not possible or have unacceptable consequences.
Diagnostics (Imaging)
Low-risk cSCC: not recommended
High-risk cSCC (factors see above): preferably by ultrasound or by CT scan for non-palpable lymph node involvement
For suspected underlying tissue involvement (bone or soft tissue): CT or MRI
Radiotherapy, post surgery
Adjuvant radiotherapy may not be offered as standard of care for cSCC with clear surgical margins.
Postoperative radiotherapy should be considered for cSCC with positive margins and for which re-excision is not possible.
Disgnostics
CT, MRI, Pet-CT to rule out distant metastatic disease
In case of a clinically or radiologically detected regional node, a fine needle aspiration cytology (FNAC) is recommended, alternatively ultrasound-guided core biopsy.
Surgery
Therapeutic lymph node dissection should be performed in patients with lymph node metastasis detected clinically or by imaging tests and confirmed with cytology or biopsy.
Elective neck dissection may be considered for metastatic cSCC within the parotid.
The extent of surgical resection is determined by the surgeon in collaboration with the multidisciplinary tumour board.
Radiotherapy, postoperative
Postoperative radiotherapy should be considered after surgical excision for cSCC with positive margins and for which re-excision is not possible.
Adjuvant radiotherapy following therapeutic lymphadenectomy should be considered in cSCC of the head and neck with regional nodal metastases and extracapsular extension.
Radiotherapy, definitive
Primary radiotherapy should be considered as an alternative to surgery for inoperable or difficult-to-operate tumours or in the absence of consent to surgical excision.
Disgnostics
lacSCC: for suspected underlying tissue involvement (bone or soft tissue): CT or MRI
CT, MRI, Pet-CT to rule out distant metastatic disease.
Medical treatment
Patients who are not candidates for curative surgery or curative radiation, should receive first-line treatment with a PD-1 antibody. Currently, PD-1 antibody cemiplimab is the only approved systemic treatment for cSCC in Europe.
Cetuximab may be used for patients with locally advanced and metastatic cSCC, who have failed to respond or are intolerant to immunotherapy. Cetuximab combined with Radiotherapy is favoured over cetuximab monotherapy.
Further treatment options are chemotherapy (platin based) and electrochemotherapy.
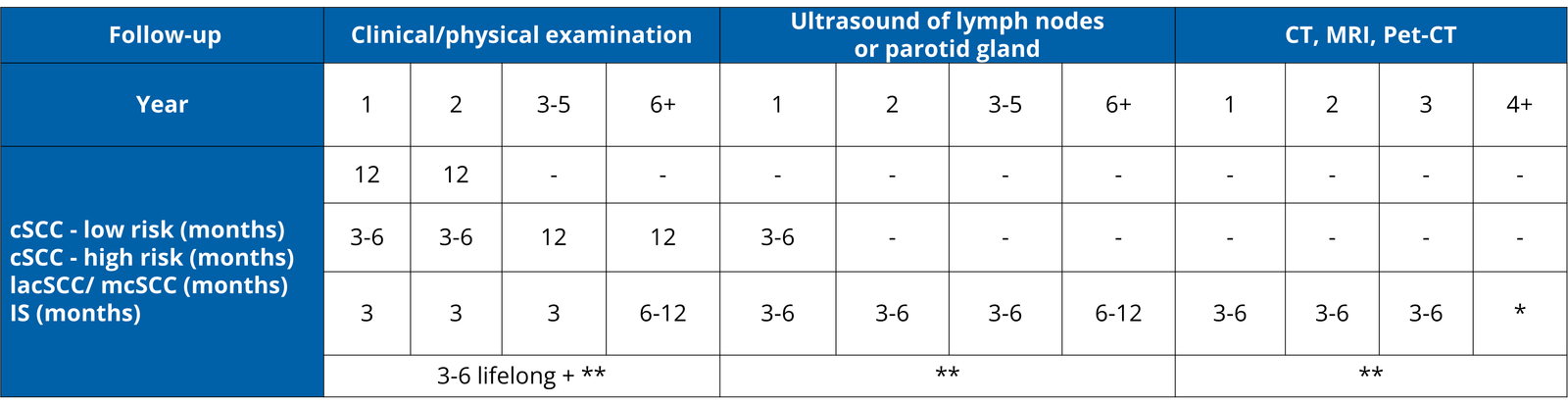
based on response, *according to characteristics of cSCC , IS-immunosuppressed
Follow-up in all patients shall include regular clinical examination, including inspection of the entire skin and inspection and palpation of the excision site, the in-transit route and the regional lymph nodes, and advice on self-skin examination.
Education about sun protection is recommended:
a. avoidance of sun bathing
b. use of protective clothing
c. regular use of sunscreens
d. avoidance of artificial UVR tanning
Adapted from
Stratigos AJ et al. European consensus-based interdisciplinary guideline for invasive cutaneous squamous cell carcinoma. Part 1: Diagnostics and prevention–Update 2023 Eur J Cancer 2023 Nov:193:113251
Stratigos AJ et al. European consensus-based interdisciplinary guideline for invasive cutaneous squamous cell carcinoma: Part 2. Treatment–Update 2023Eur J Cancer 2023 Nov:193:113252
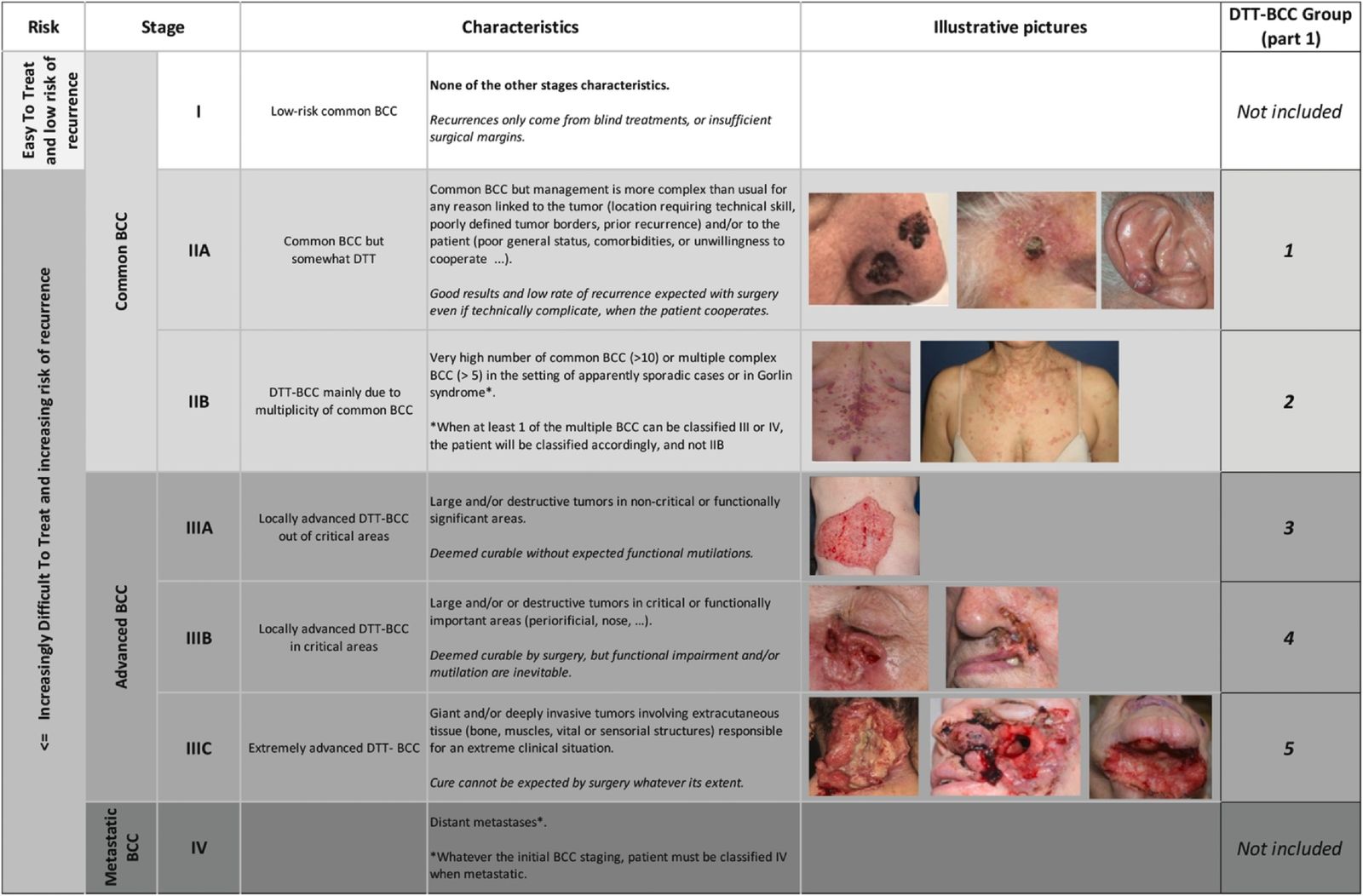
Fig. EADO classification and staging for BCC. BCC, basal cell carcinoma; EADO, European Association of Dermato-Oncology. DDT, difficult-to-treat
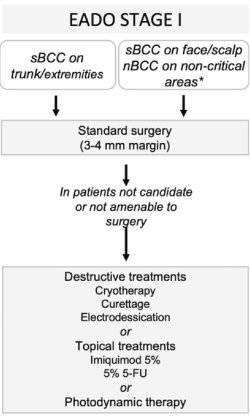
*critical areas: central face, eyelids, eyebrows, nose, lips, chin, ear, periauricular; *aggressive subtypes: micronodular, morpheaform, infiltrative, metatypical (basosquamous carcinoma); ***in cases of multiple sporadic BCCs, radiotherapy might be used to treat one or a few large tumors.
Fig. Treatment algorithm for BCC. BCC, basal cell carcinoma; EADO, European Association of Dermato-Oncology; 5-FU, 5-fluorouracil; nBCC, nodular subtype of BCC; sBCC, superficial BCC.
Surgery
Surgery followed by histopathological confirmation is standard of care.
3-4-mm safety margin for standard excision and 2D histology.
Narrow margins: If R0 is reported, re-excision is not required.
Topical treatment/ Destructive treatment
Patients not candidate or not amendable to surgery:
Superficial and small nodular BCC: Imiquimod 5% or
Superficial BCC: 5% 5-Fluorouracil (inferior to imiquimod and non-inferior to MAL-PDT) or
Photodynamic Therapy with 5-ALA or MAL in combination with red light (inferior to imiquimod 5%) or
Cryotherapy, Curettage or Eletrodissection
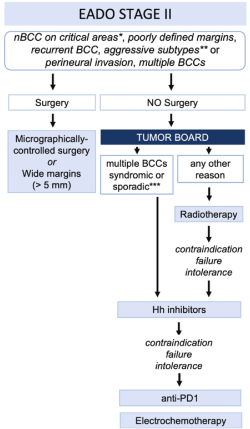
*critical areas: central face, eyelids, eyebrows, nose, lips, chin, ear, periauricular; *aggressive subtypes: micronodular, morpheaform, infiltrative, metatypical (basosquamous carcinoma); ***in cases of multiple sporadic BCCs, radiotherapy might be used to treat one or a few large tumors.
Fig. Treatment algorithm for BCC. BCC, basal cell carcinoma; EADO, European Association of Dermato-Oncology; 5-FU, 5-fluorouracil; nBCC, nodular subtype of BCC; sBCC, superficial BCC.
Surgery
- Micrographically controlled surgery (3D) in high-risk BCC (recurrent, aggressive subtypes, location in critical anatomical sites, poorly defined margins) is recommended.
- 5mm safety margin in high risk-BCC (recurrent, aggressive subtypes, location in critical anatomical sites, poorly defined margins) and 2D histology.
Incompletely excised BCC lesions (R1 or R2), shall be re-excised.
Radiotherapy
In patients who are not candidates for surgery or decline surgery.
After surgery: in case of incomplete resection with microscopic (R1) or macroscopic (R2) residual tumour, when the multidisciplinary tumour board does not consider follow-up or a wide surgical excision as the best option.
Medical treatment (after discussion in multidisciplinary tumor board in patients with multiple BCC syndromic or sporadic)
Hedgehog inhibitors
Anti-PD1 immunotherapy as second-line treatment in patients who progress or have contraindications to hedgehog inhibitors
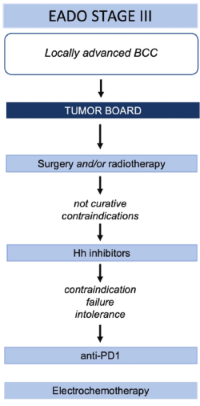
Fig. Treatment algorithm for BCC. BCC, basal cell carcinoma; EADO, European Association of Dermato-Oncology; 5-FU, 5-fluorouracil; nBCC, nodular subtype of BCC; sBCC, superficial BCC.
Diagnostics
Physical examination
Imaging (CT, MRI) identifying invasion of deep structures (bone, muscles, vessels) and perineural invasion.
Radiotherapy
In patients who are not candidates for surgery or decline surgery.
After surgery: incomplete resection with microscopic (R1) or macroscopic (R2) residual tumour, when the tumour board does not consider follow-up or a wide surgical excision as the best option
Medical treatment
Hedgehog inhibitors
Anti-PD1 immunotherapy as second-line treatment in patients who progress or have contraindications to hedgehog inhibitors
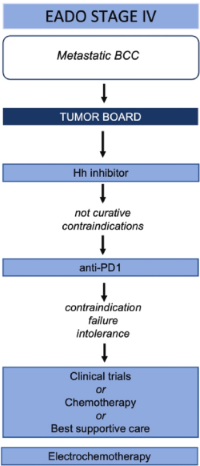
Fig. Treatment algorithm for BCC. BCC, basal cell carcinoma; EADO, European Association of Dermato-Oncology; 5-FU, 5-fluorouracil; nBCC, nodular subtype of BCC; sBCC, superficial BCC.
Radiotherapy
In patients who are not candidates for surgery or decline surgery.
After surgery: incomplete resection with microscopic (R1) or macroscopic (R2) residual tumour, when the tumour board does not consider follow-up or a wide surgical excision as the best option
Medical treatment
Hedgehog inhibitors
Anti-PD1 immunotherapy as second-line treatment in patients who progress or have contraindications to hedgehog inhibitors
Follow-up is recommended in patients with BCC in 3–12 monthly intervals according to the risk category.
All BCC patients: one visit after treatment for skin examination and to discuss their diagnosis and treatment, sun-protection measures, importance of self-monitoring
Patients who are at high risk for recurrent lesions (i.E. recurrent BCC, history of multiple BCCs): follow-up every 12 months for 3–5 years (if not lifelong).
Difficult-to-treat or advanced BCC: multidisciplinary tumor board for individual follow-up.

The diagnosis of Gorlin syndrome is established with two major diagnostic criteria and one minor diagnostic criterion or one major and three minor diagnostic criteria.
Genetic testing for germline mutations in the Hedgehog pathway can be offered in selected cases.
Treatment of patients with Gorlin syndrome requires a multidisciplinary approach. In selected patients, treatment with Hedgehog inhibitors can be considered.
Close surveillance and regular skin examinations (total body skin examination including scalp und genitalia) with dermatoscopy: annually starting at the age of 10 years in carries of PTCH1 variants and at the age of 20 years in SUFU variant carriers, and then every 4–6 months.
Adapted from and further Information
Peris K. et al. European consensus-based interdisciplinary guideline for diagnosis and treatment of basal cell carcinoma—update 2023, EJC 192 (2023) 113254
Surgery /Safety margin (SM)
Complete excision with clinical safety margin: SM 1 cm – followed by adjuvant radiotherapy
If adjuvant radiotherapy is not possible: SM up to 2 cm
Preoperative diagnostics (Clinical and imaging)
Full body skin examination including clinical examination of all main lymph node basins
Imaging: Ultrasound of regional lymph node basin, whole body imaging (i.E. contrast-enhanced CT-scan of neck/thorax/abdomen/pelvis), if available FDG PET/CT.
In asymptomatic patients no routine imaging of the brain.
Sentinel lymph node biopsy (SLNB)
Recommended for stage I/II patients, in the absence of clinical or imaging evidence for nodal or distant metastases
In patients not eligible SLNB in the head and neck region: radical RT can be suggested (SLNB is not performed and the only treatment is RT)
Radiotherapy (RT)
MCC cells are highly sensitive to radiation.
Adjuvant RT to the primary tumour bed should be performed within 8 weeks of surgical excision.
Palliative radiotherapy alone: frail patients to treat primary tumours and/or nodal involvement when surgery is not feasible
(SLN + or lymph node metastases detected clinically or by imaging)
CLND/TLND/Imaging/Radiotherapy
a. Microscopic nodal disease (positive SLN): adjuvant RT alone (50-55 Gy)
or eventually combined with CLND
CLND alone is an option in case adjuvant RT is not possible
b. Lymph node metastases (detected clinically or by imaging): Therapeutic lymph node dissection (TLND)
Imaging: whole body imaging + Brain MRI for stage ≥ III or symptomatic patient
Adjuvant RT should be discussed in a multidisciplinary board after CLND or TLND
Frail patients: palliative radiotherapy alone to treat primary tumours and/or nodal involvement when surgery is not feasible, decision in interdisciplinary tumour board
Clinical trials
If available and appropriate
Radiotherapy
In advanced patients with MCC, RT is routinely performed with palliative intent for symptomatic lesions either alone or combined to systemic therapies
Medical treatment
Immunotherapy: Immunocompetent patients with locally advanced or metastatic MCC (surgery no feasible) shall receive anti PD-(L)1 –based immunotherapy as first line treatment.
Chemotherapy (platinum-based drugs, etoposide, taxanes, anthracyclins, either alone or in various combinations): can be used when patients fail to respond, are intolerant or present contraindication to anti-PD-(L)1 immunotherapy, or when immunotherapy or clinical trials are not available. For patients in good performance status i.E. a combination of platinum and etoposide (Cisplatin 60-80 mg/m2 IV on day 1 plus etoposide 80-120 mg/m2 IV on days 1-3 every 21-28 d or Carboplatin AUC 5 IV on day 1 plus etoposide 80-100 mg/m2 IV on days 1-3 every 28 d)
Follow-up
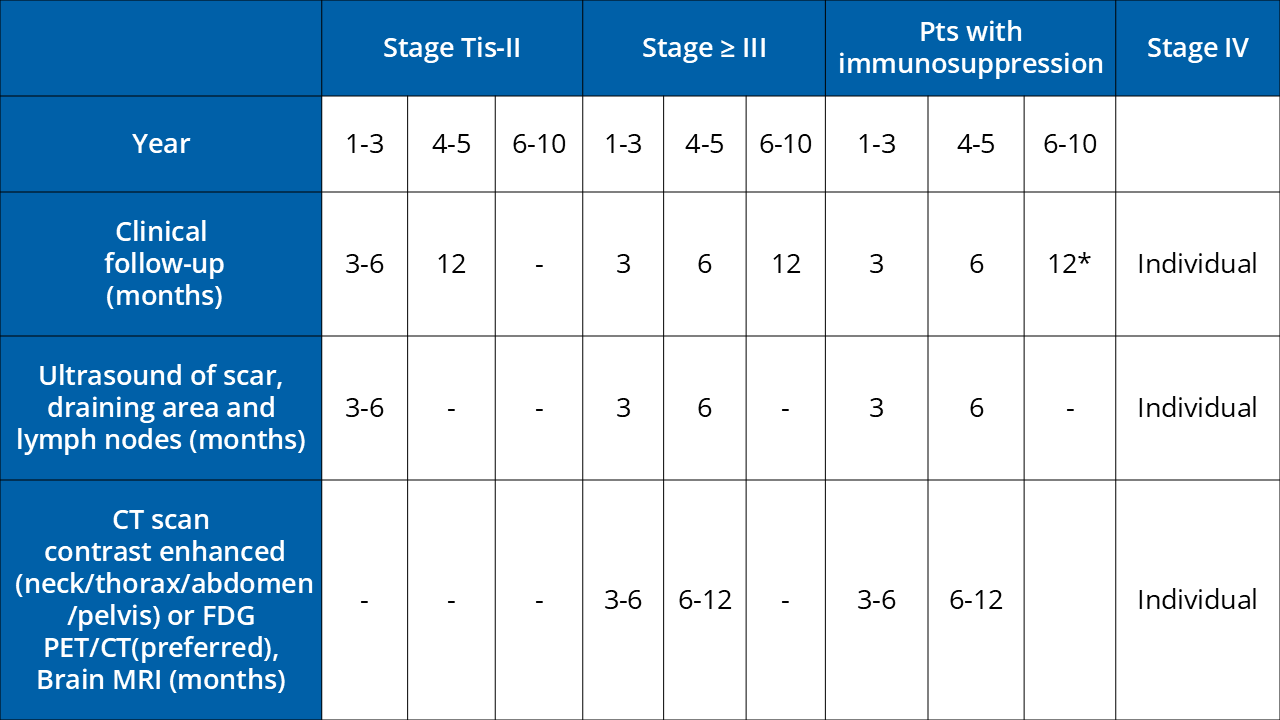
*If no subsequent cancers have occurred
Further Informationen
Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline e Update 2022, Gauci M-L et al. European Journal of Cancer 171 (2022) 203-231
Clinical Diagnosis
Clinical suspicion: Biopsy for histopathology before extensive surgery
Pathology
Pathology reports: presence or absence of high mitotic rates and fibrosarcomatous changes (FS).
Molecular Analysis (incl. FISH)
Should be performed when possible. When this technology is not available: expert pathology opinion
Diagnostics (Imaging)
DFSP: not routinely (distant metastases are extremely rare)
Preoperative for estimation of tumour (i.E. large tumours, difficult situation) extension: MRI - contrast enhanced
Clinical suspicion of metastasis, recurrent disease, and FS-DFSP: CT-scan
Surgery /Safety margins (SM)
Complete resection (without SM): Any micrographically controlled surgery based on horizontal sections (Mohs surgery) or sectioning allowing the entire peripheral and deep margin assessment, 3D-surgery.
Wide local excision (with 2-3 cm SM lateral and resection of underlying fascia): If no variant of micrographically controlled surgery is available.
Reconstruction of defects: avoid flaps and favour direct closure, skin grafting or secondary intention healing to facilitate early detection of local recurrences.
Cave: Recurrent DFSP tumours should be resected whenever possible using micrographically controlled surgery.
Radiotherapy
DFSP (R0) non-transformed tumours: no
DFSP (close margins, recurrent tumours, aggressive histological subtypes or as a salvage treatment in the presence of positive margins: may be considered
Fibrosarcomatous transformed DFSP (FS-DFSP)
Multidisciplinary specialized soft-tissue sarcoma tumour board including soft-tissue sarcoma expert(s).
CT scan of the thorax, abdomen, pelvis and lymph node ultrasonography.
Complete surgical excision with micrographically controlled surgery. If no variant of micrographically wide local excision (with 2-3 cm SM lateral and resection of underlying fascia)
Radiotherapy
Decision in Multidisciplinary sarcoma tumour board
Consider in primary inoperable tumours radiation therapy with conventional fractionation (2 Gy/day, 5 days per week) with a dose range from 50 up to 70 Gy, usually a total dose of 60 Gy (microscopic residual tumour) to 70 Gy (macroscopic residual tumour).
Systemic treatment
EMA-approval for Imatinib 400 to 800mg/d: inoperable primary tumours, locally advanced and recurrent cases, and metastatic DFSP
Chemotherapy: not effective & not recommended.
Immune checkpoint inhibition: limited data
Follow-up
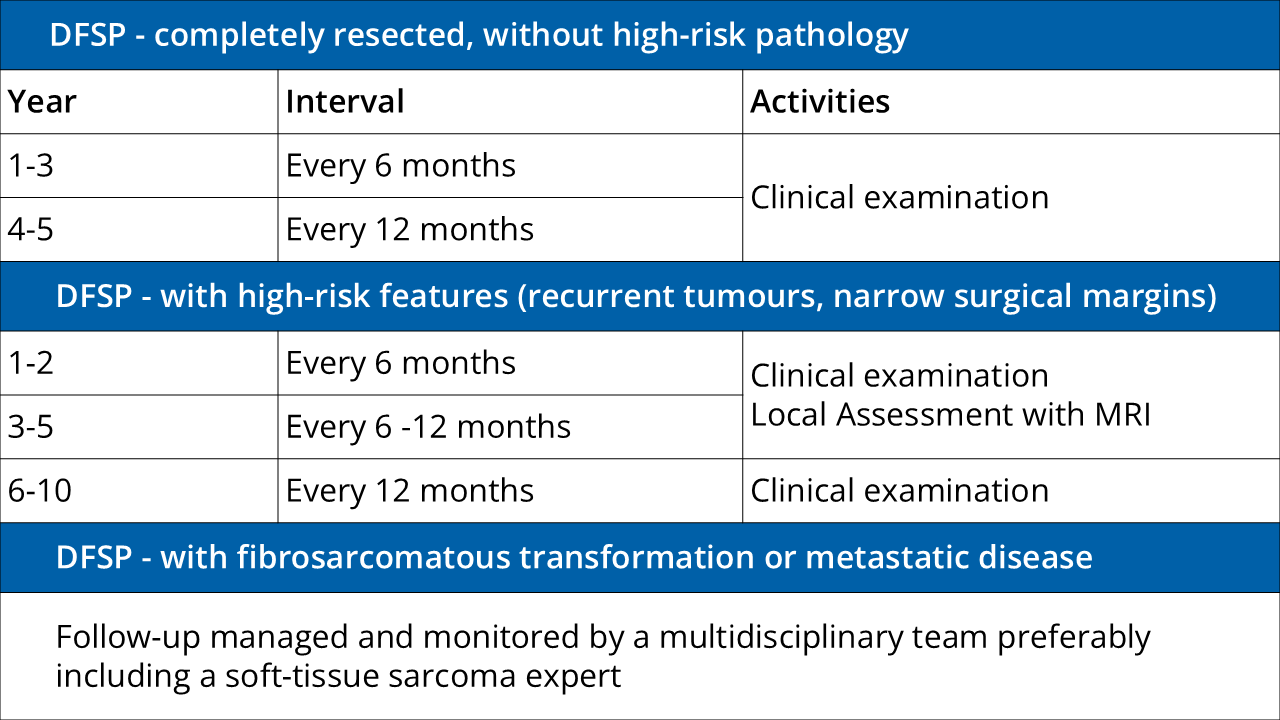
Further Information
Adapted from: Diagnosis and treatment of dermatofibrosarcoma protuberans. European interdisciplinary guideline – update 2024, EJC 218(2025) 115265.
Surgery/safety margin
AFX: micrographically controlled surgery (MCS) or clinical safety margin of at least 0.5 cm
PDS: broad safety margin, if possible 2 cm with MCS; safety margin may be adapted to the anatomical situation if required
Diagnostic (Imaging)
AFX: no
PDS: locoregional lymph node sonography
Or if locoregional spreading is suspected or detected.
Locoregional cross-sectional imaging: non-movable tumors or suspected deep infiltration.
Adjuvant Radiotherapy (R0)
AFX: no
PDS without a safety margin: to reduce the rate of locoregional recurrence may be considered.
Radiotherapy
In cases of inoperability or incomplete resection of the tumor, irradiation of the tumor area may be considered.
Medical treatment
Noperable or metastasized PDS requires individual therapeutic decisions. Treatment with a checkpoint inhibitor appears to be a promising option but requires off-label use.
Follow-up
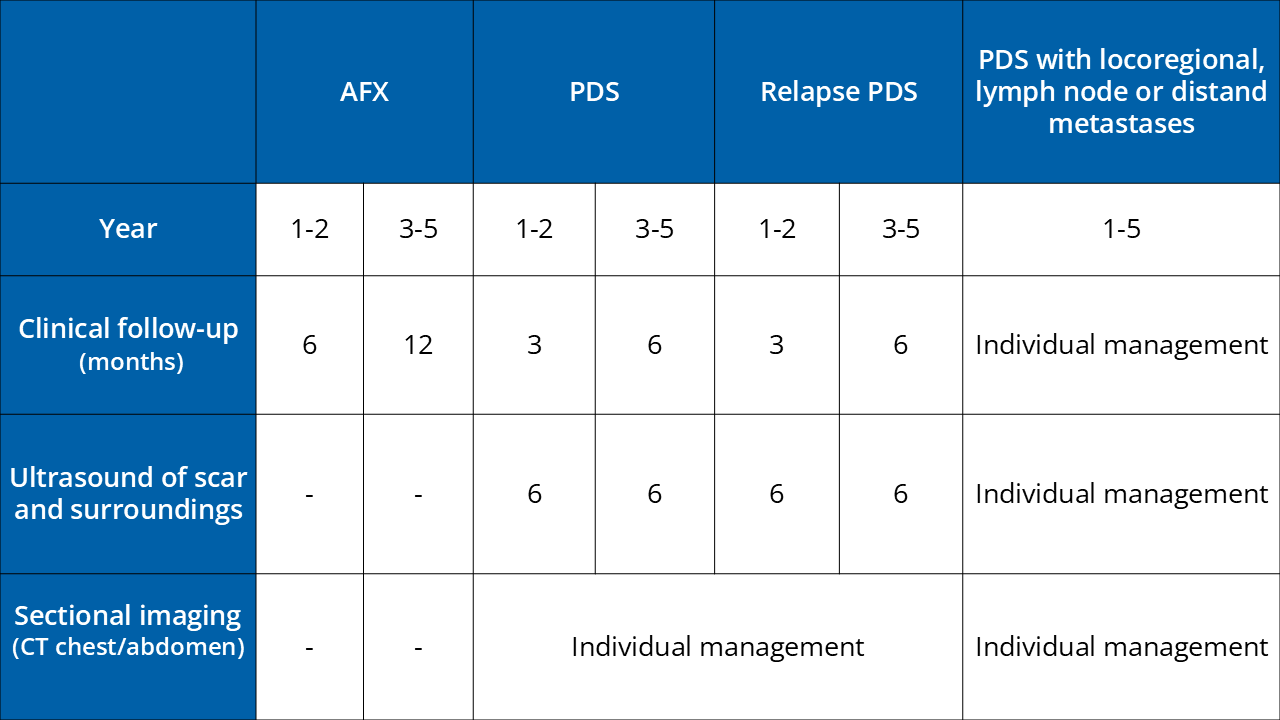
Further Information
Adapted from S1-guideline atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma (PDS) AWMF 032/057;
Subtypes Kaposi´s Sarcoma (KS)
Classic KS
Endemic KS
Epidemic KS in HIV positive patients
Iatrogenic KS
Staging workup
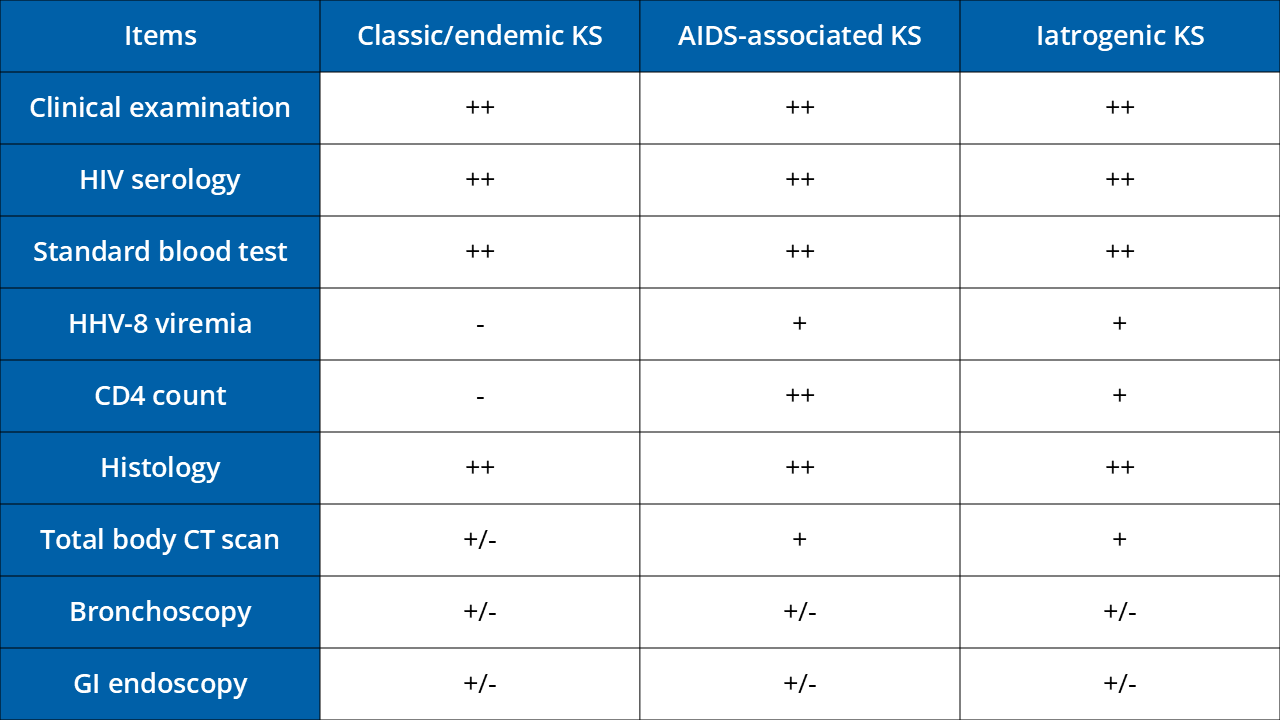
CT, computerised tomography; KS, Kaposi’s sarcoma; HIV, human immunodeficiency virus; US, ultrasound; GI, endoscopy; HHV-8, human herpesvirus-8.
- : usually not +: usually yes ++: mandatory +/- : according to symptoms
Treatment
Radiotherapy is most efficient treatment for localised KS. 30-36 Gy in 2- or 3-Gy daily fractions using low-energy photons and/or electrons. Information about possible risks of out-of-field recurrence and of radiotherapy-induced skin toxicity like telangiectasia, hyperpigmentation, skin atrophy and fibrosis.
Surgical Excision in few well-defined lesions. Caution: high recurrence rate and repeated excisions can cause functional impairment.
Cryosurgery and laser in superficial lesions, response rate 80-90%, information about risk of sequelar hypopigmentation to patients.
Topical treatments/ local or intralesional chemical or immunemodifying agents:
Intralesional Vincristin or Vinblastin
Electrochemotherapy with Bleomycin
Imiquimod
Topical 9-cis-retinoid acid (Alitretinoin gel 0.1%) – currently in EU not available
Medical treatment in aggressive forms characterised by lymph node and/or visceral involvement, severe oedema, local complications or rapid extension: liposomal anthracyclines and, less frequently, weekly taxanes. Or low dose interferon alpha in younger, healthy patients (<70 years old and normal cardiac function).
Iatrogenic KS: Tapering down immunosuppressive therapy to the lowest possible level and switching to mammalian target of rapamycin (m-TOR) inhibitors such as sirolimus.
HIV-related KS regresses with combination antiretroviral therapy. Chemotherapy is recommended for patients with poor prognosis or rapidly progressive disease.
Follow Up
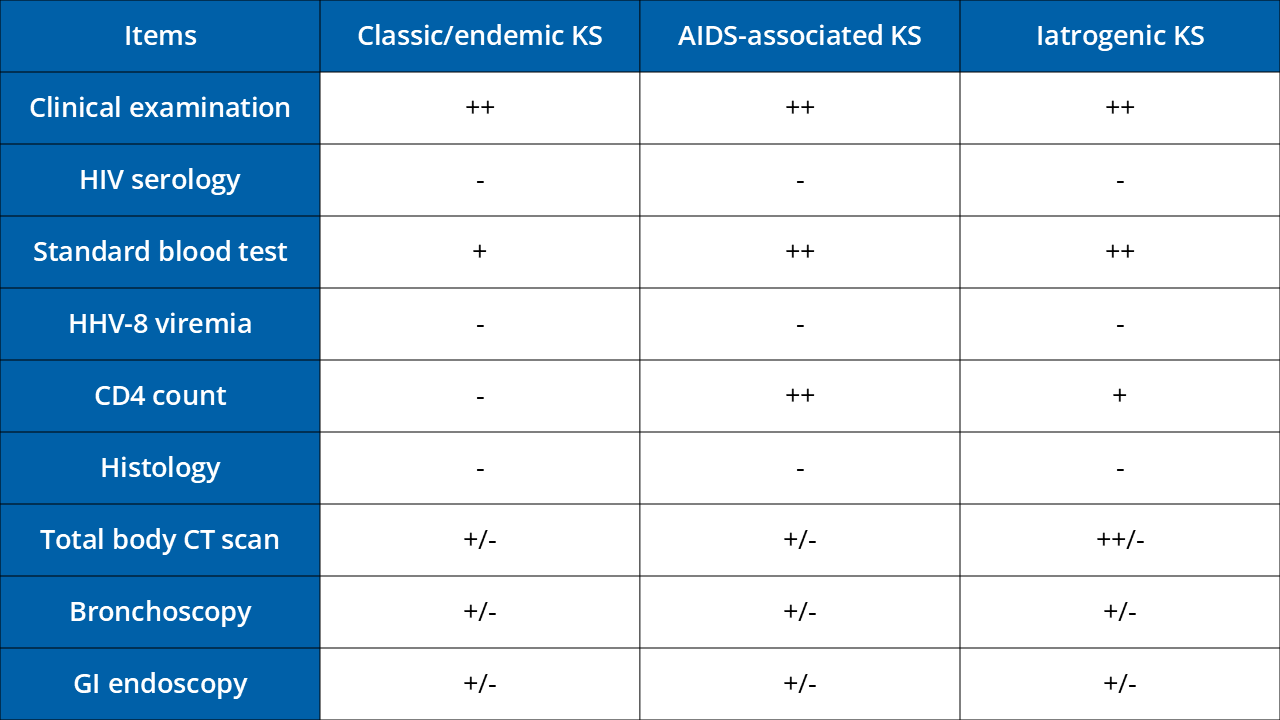
Further information
Lebbe C. et al. Diagnosis and treatment of Kaposi's sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC) EJC 2019 DOI: 10.1016/j.ejca.2018.12.036
Surgical treatment/safety margin (SM)
Dermal LMS: Primarily microscopically controlled R0, extension of the SM to 1 cm
Subcutaneous LMS: Primarily microscopically controlled R0, if possible extension of the SM to 2 cm, or rather to the fascia (or according to an interdisciplinary tumor conference)
Diagnostics (imaging)
None in standard cases.
In case of suspicion or evidence of locoregional metastasis: ultrasound of regional lymph nodes
In the case of non-displaceable tumours/ suspected deep infiltration: locoregional CT or MRI
Adjuvant radiation therapy
LMS R0 and small tumours: none
LMS R1 or R-2 resection, small safety margin or large tumours
(LMS > 5cm): recommended
Radiotherapy:
In palliative, inoperable situations: therapeutic radiotherapy
Medical treatment:
Individual therapy decision
E.g. doxorubicin or anthracycline-based combination therapy (in case of rapid progression)
Follow-up care:
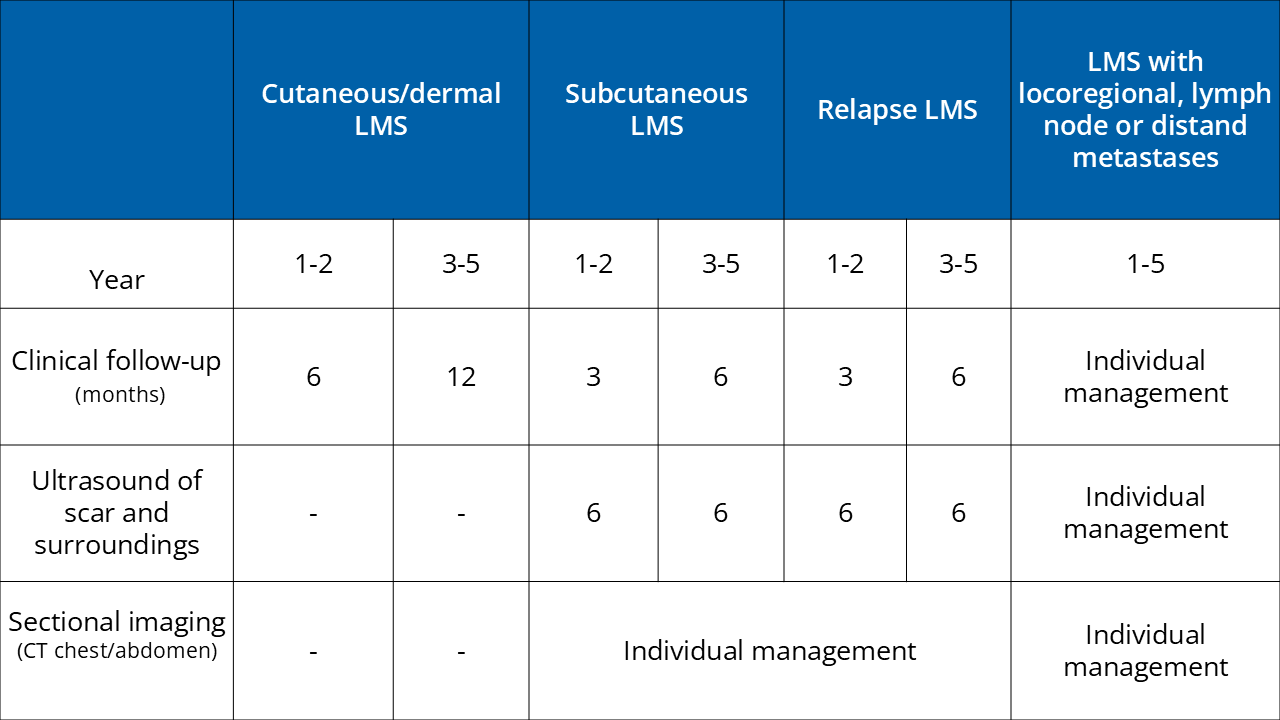
Further information:
Reference: Helbig et al. S1-guideline cutaneous and subcutaneous leiomyosarcoma https://doi.org/10.1111/ddg.14989
Idiopathic or classic angiosarcoma of elderly patients
Angiosarcoma secondary to lymphoedmea
Postradiation angiosarcoma
Epithelioid angiosarcoma
Surgical treatment
Primary excision with the aim of R0 (microscopically controlled) while preserving function. Avoid delays in subsequent radiotherapy.
Alternative mass reduction (R1) and adjuvant radiotherapy or primary radiochemotherapy.
Diagnostics (imaging)
Individualized: Lymph node ultrasound, CT or MRI, alternatively PET-CT (for additional information including extension in the soft tissue for surgical planning)
Adjuvant radiotherapy: postoperative radiotherapy at the primary site with individualized appropriate safety zone recommended.
Medical treatment
- Primary radiochemotherapy or
- Anthracyclines and taxanes, immune checkpoint inhibitors (case reports)
Electrochemotherapy as palliative approach for local control


Further Inofmation: S1-Guideline Cutaneous Angiosarcomas - Update 2021
Vogt T. et al, J Dtsch Dermatol Ges 2021 Dec;19(12):1801-1812
Diagnostics
Clinical examination of the whole body
Clinical examination of the regional lymph nodes
Periocular: presentation to ophthalmologist
Surgery – resectable sebaceous carcinoma
Safety Margin (SM): No SM recommended for microscopically controlled surgery (MKC)
Periocular without MKC: 3-5 mm SM
Extraocular without MKC: wide local excision with at least 1 cm SM
If peri-/extra-ocular sebaceous gland carcinoma with pagetoid/surface spread: tumour conference, if necessary mapping biopsies for diagnostic workup of spread
Sentinel lymph node biopsy (SLNB)
Discussion and individual decision in interdisciplinary tumour board recommended. Prognostic and therapeutic relevance unclear. Metastasis to regional lymph nodes rare (periocular 15%, extraocular <1%).
Periocular: can be considered for tumours ≥T2c (infiltration of the entire width of the eyelid margin)
Extraocular: SLNB can be considered for risk factors (localisation lip, ear, ≥T2c, poor differentiation, pagetoid growth, perineural invasion, immunosuppression)
Complete lymph node dissection (CLND) / therapeutic lymph node dissection (TLND)
Discussion and individual decision in the interdisciplinary tumour board (ITB) recommended.
Periocular and SLN +: CLND & ITB
Extraocular and SLN +: no CLND & ITB
In case of clinical suspicion of LK metastasis: TLND & ITB
Diagnostics (imaging)
Regional Lymph nodes: not routinely using sonography or cross-sectional imaging, only in recurrent sebaceous gland carcinoma, extensive tumours and suspected metastasis as well as in periocular ≥T2c, poor differentiation, pagetoid growth, perineural invasion
Potential distant metastases: by means of cross-sectional imaging only for LK metastases
Radiotherapy
Adjuvant 45-60Gy, therapeutic 56-70Gy,
Extraocular adjuvant: after individual assessment for locally advanced tumours, R1/R2, scarce SA <5mm, PNI, local recurrence, in the case of LK metastases
Extraocular therapeutic: in case of inoperability, refusal of surgery or palliative situation
Periocular adjuvant: Benefit not clarified, possibly for R1, recurrence, low differentiation, PNI
Periocular therapeutic: indications inoperability, recurrence, option for extensive orbital involvement, tumours <10mm
CAVE: relevant ocular toxicity with upper eyelid radiotherapy, consider high-dose brachytherapy as a boost plus ERBT if necessary
Periocular and SLN+: possibly an alternative to CLND
Lmph node metastases: Consider radiotherapy after TLND
Palliative: possible option
Local treatment
Periocular with pagetoid or intraepithelial spread: as monotherapy or in combination with surgery using cryosurgery and/or mitomycin locally
Medical treatment
Locally advanced or metastasized sebaceous carcinoma: conventional protocols with chemotherapy (e.g. 5-FU + platinum-based) only case series, neoadjuvant concepts exists
Promising option: PD-1 inhibitors (especially extraocular tumours), trastuzumab (if HER2 detectable)
Molecular diagnostics for potential targets for targeted therapy if necessary: RAR-β, androgen receptor, mTOR, EGFR, HER2, Pi3K
Follow-up
Local recurrences frequent (>10%), in 5.5-6.7% also with distant metastases.

Muir-Torre syndrome
If ≥2 points are present in the risk score: genetic diagnostics.
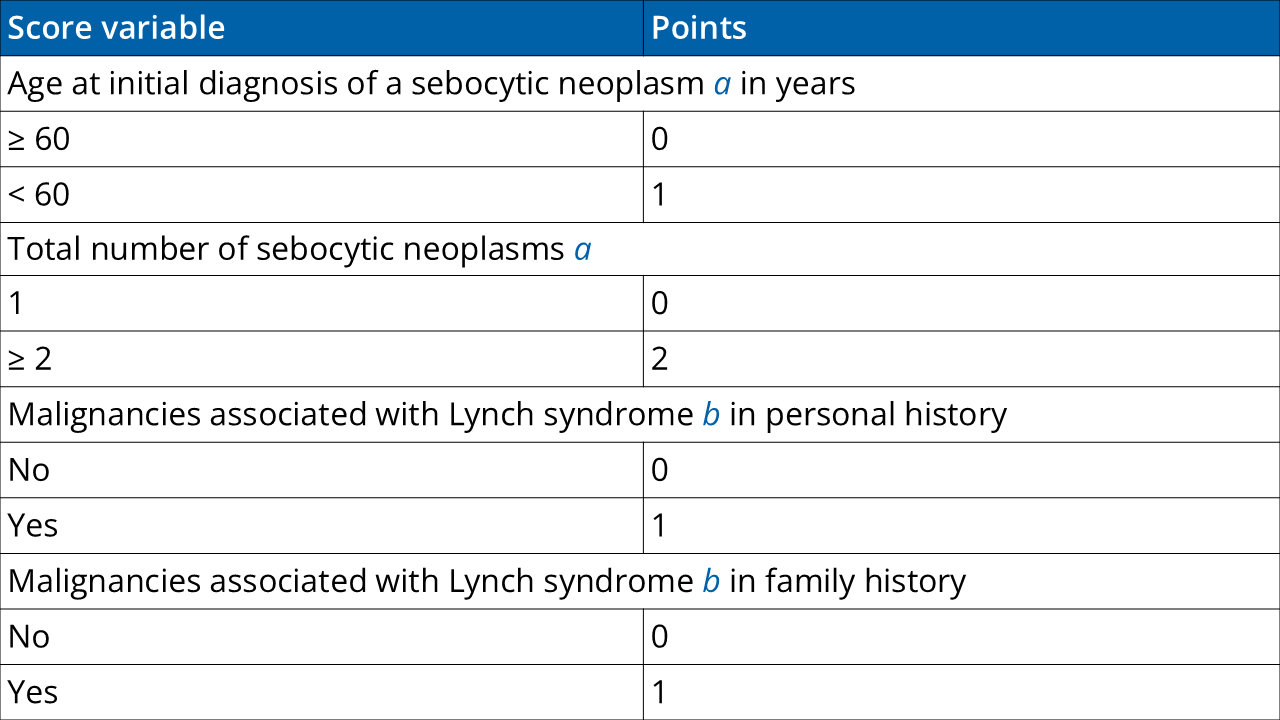
The total score is the result of the addition of the points obtained for the four score variables. Therefore, total score values from 0–5 can be obtained. A score of ≥ 2 has a sensitivity of 100% and a specificity of 81% for the prediction of a germline mutation in one of the Lynch syndrome mismatch repair genes.
a Sebocytic neoplasms include sebaceous gland adenoma, sebaceous gland epithelioma, keratoacanthoma, and sebaceous gland carcinoma. Sebaceous gland hyperplasia is not considered as MTS-associated. Accordingly, a patient presenting only with sebaceous gland hyperplasia receives no point.
b Malignancies associated with Lynch syndrome include colorectal carcinomas, endometrial carcinomas, ovarian carcinomas, gastric carcinomas, small intestine carcinomas, urothelial cell carcinoma, (renal pelvis/ureter), and bile duct carcinomas.
Further information
Adapted from S1-Guideline Sebaceous Carcinoma, Utikal J. et al First published: 28 April 2024 https://doi.org/10.1111/ddg.15405
